Several thousand years ago, a mysterious machined aluminum object entered the archaeological record in what is now Romania, on the banks of the River Mures near the town of Aiud. Or maybe it was a few hundred years, or maybe a quarter million—or maybe it was in the middle of the 20th century. I strongly suspect the latter dating is correct.
Several thousand years ago, a mysterious machined aluminum object entered the archaeological record in what is now Romania, on the banks of the River Mures near the town of Aiud. Or maybe it was a few hundred years, or maybe a quarter million—or maybe it was in the middle of the 20th century. I strongly suspect the latter dating is correct.
I wrote a brief note about this mysterious object, the so-called Wedge of Aiud, some time back; to my surprise, that blog post continued to get comments and queries, some of them quite impassioned. I think it’s worth revisiting.
Recap: the object was allegedly recovered in about 1973, 10 metres down in a sandpit/construction excavation near the river, in the company of two bones identified as belonging to a mastodon (or hairy rhino in some versions). Analysis showed the wedge to be composed of an Al-Cu alloy with various additives, though several tests over the decades produced varying percentages. A corrosion layer of about 1mm thickness was claimed to indicate an age between 400 and 250,000 years—but processes for refining aluminum were developed only within the last two centuries.
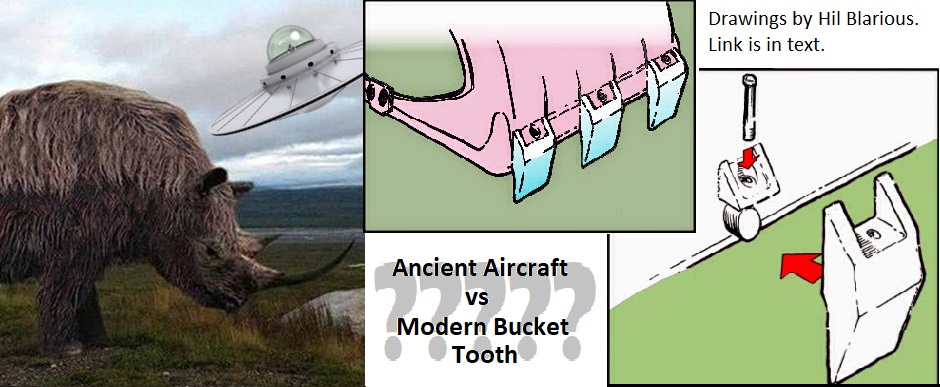
That is why the object is claimed to be an OOPART—an out-of-place artifact—and its believers usually interpret it as part of the landing gear of a prehistoric VTOL aircraft or (inevitably) a flying saucer which had an unfortunate encounter with a mastodon. To some of us, though, it looks more like a discarded bucket tooth from a mid-twentieth century clamshell excavator, cast from poor-quality 2000-series aluminum alloy (aka duralumin), and found in a pit that was most likely being dug by a clamshell excavator. (The blogger Hil Blairious has produced a very plausible design of how the object could have been attached to the bucket.)
A major sticking point seems to be the depth of the “patina” on the object’s surface—that is, the 1mm layer of corrosion products, which is usually described as “thick.” The layer is described only as aluminum oxide, though it is unclear to what extent it was separately tested. The photos show extensive pitting corrosion, to which Al-Cu alloys are particularly prone; and the original laboratory analysis apparently made some cryptic mention of “the allied elements partially regaining their own  structures,” which sounds suspiciously like the effect of galvanic corrosion. At first glance, this would be surprising, since galvanic corrosion tends to involve contact between metals of differing electrical potential, but I’ll return to that later.
structures,” which sounds suspiciously like the effect of galvanic corrosion. At first glance, this would be surprising, since galvanic corrosion tends to involve contact between metals of differing electrical potential, but I’ll return to that later.
The huge range of suggested dates, from 400 to 250,000 years, should itself serve as a red flag, but it is not the only dubious aspect of this claim. Another is that none of these suggested dates was presented with any documentation or any statement of methodology. To the best of my knowledge, there are still few techniques for dating archaeological metal, and none involves aluminum or aluminum alloys. Basing an age estimate solely on the corrosion-layer thickness ignores the huge variability in the rate at which aluminum alloys will corrode, depending on composition and conditions like ambient moisture, pH, salinity, temperature, and surrounding materials. There is no simple coefficient you can apply. And in fact, there is no reason to think the degree of corrosion on the Aiud Wedge is anything extraordinary, to the point that it would require thousands or even hundreds of years to build up.
This last has to do partly with the nature of the alloy. Pure aluminum is indeed  resistant to corrosion, because its surface quickly and spontaneously forms a very thin protective film of aluminum oxide, only nanometres thick; this is this property that gives aluminum its reputation for corroding at a glacial pace, if at all, and is probably one reason why the 1mm coating on the Aiud wedge is considered evidence of great age.
resistant to corrosion, because its surface quickly and spontaneously forms a very thin protective film of aluminum oxide, only nanometres thick; this is this property that gives aluminum its reputation for corroding at a glacial pace, if at all, and is probably one reason why the 1mm coating on the Aiud wedge is considered evidence of great age.
But aluminum alloys are a different matter. Unprotected, they can and do corrode rapidly under a wide variety of circumstances, such that a mm of pitted corrosion product is nothing unusual. In the case of Al-Cu alloys, the addition of copper makes the fabric stronger, but also makes it peculiarly vulnerable to corrosion. Where this would be problematic—say, when it is being used in aircraft components—the problem can be alleviated by other additions, such as magnesium, or by protecting the surface with a thin coat of purer aluminum. Notably, none of the assays conducted on the Aiud object found any significant magnesium content. (See here for a summary and discussion of the various analyses.)
So what we have is an object made of an aluminum alloy particularly vulnerable to corrosion, but strong enough for rough work. Its provenience and the circumstances of its discovery are unclear—another red flag—but the narrative consistently places it about 10m down in an industrial excavation close to the river, in a wet matrix. Significant variables that would affect the rate of corrosion are unknown, such as pH, salinity, and soil composition; however, there is an absolutely critical known factor that I have not seen taken into account in any previous discussion: the effect of heavy metals pollution.
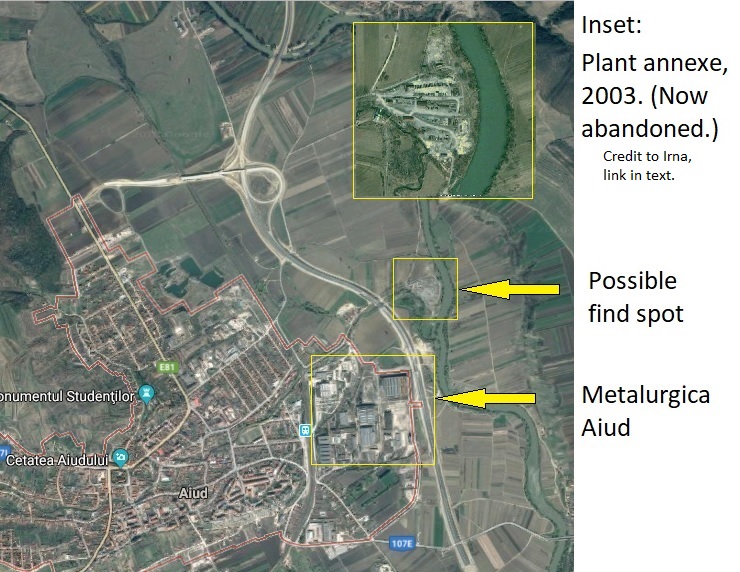
Aiud was a major centre of metallurgical heavy industry between 1931 and 2013, when the great factory complex of Metalurgica Aiud shut down for good. The works are situated on the eastern outskirts of the town, virtually on the bank of the River Mures, in roughly the area mentioned as the object’s source; the blogger Irna plausibly suggests the best candidate is an offshoot of the plant a little to the northeast, now abandoned. Interestingly, the Metalurgica Aiud expanded its facilities in the 1970s, the period when the object was found; but at any rate, a find-spot in the vicinity of the works is more than likely.
In its heyday, Metalurgica Aiud employed about half the residents of Aiud (the infamous prison probably accounted for a good portion of the rest). But the town’s prosperity came at the cost of heavy, lingering soil and groundwater pollution, a heritage that leaves it even now with levels of lead, copper, cadmium, and zinc that far outstrip the legal maxima, partially attributed to historic pollution from the plant. Whatever wet matrix the Aiud object was found in would have been powerfully contaminated with heavy metals, salts, and other industrial pollutants. The abysmal record of Soviet-era industrialization in this regard is well known. Which brings us back to the corrosion layer:
Heavy metal ions such as copper and mercury are very aggressive toward the pitting corrosion of aluminum alloys. The aluminum reduces the ions of copper, mercury, lead, etc. These ions can plate out on the aluminum and form localized galvanic cells with the aluminum becoming the anode and the heavy metal becoming a very effective cathode.
This galvanic deposition corrosion bumps up and accelerates the process, in the form of extensive rapid pitting plus a heavy buildup of corrosion products and deposits of the heavy metal which is acting as the cathode—which sounds like a pretty fair description of the Aiud Wedge. And it is not a process measured in centuries or millennia, but in months or years.
To summarize: we have an aluminum alloy known to be vulnerable to corrosion, found in a wet matrix contaminated with heavy metals known to accelerate corrosion. Where is the mystery?